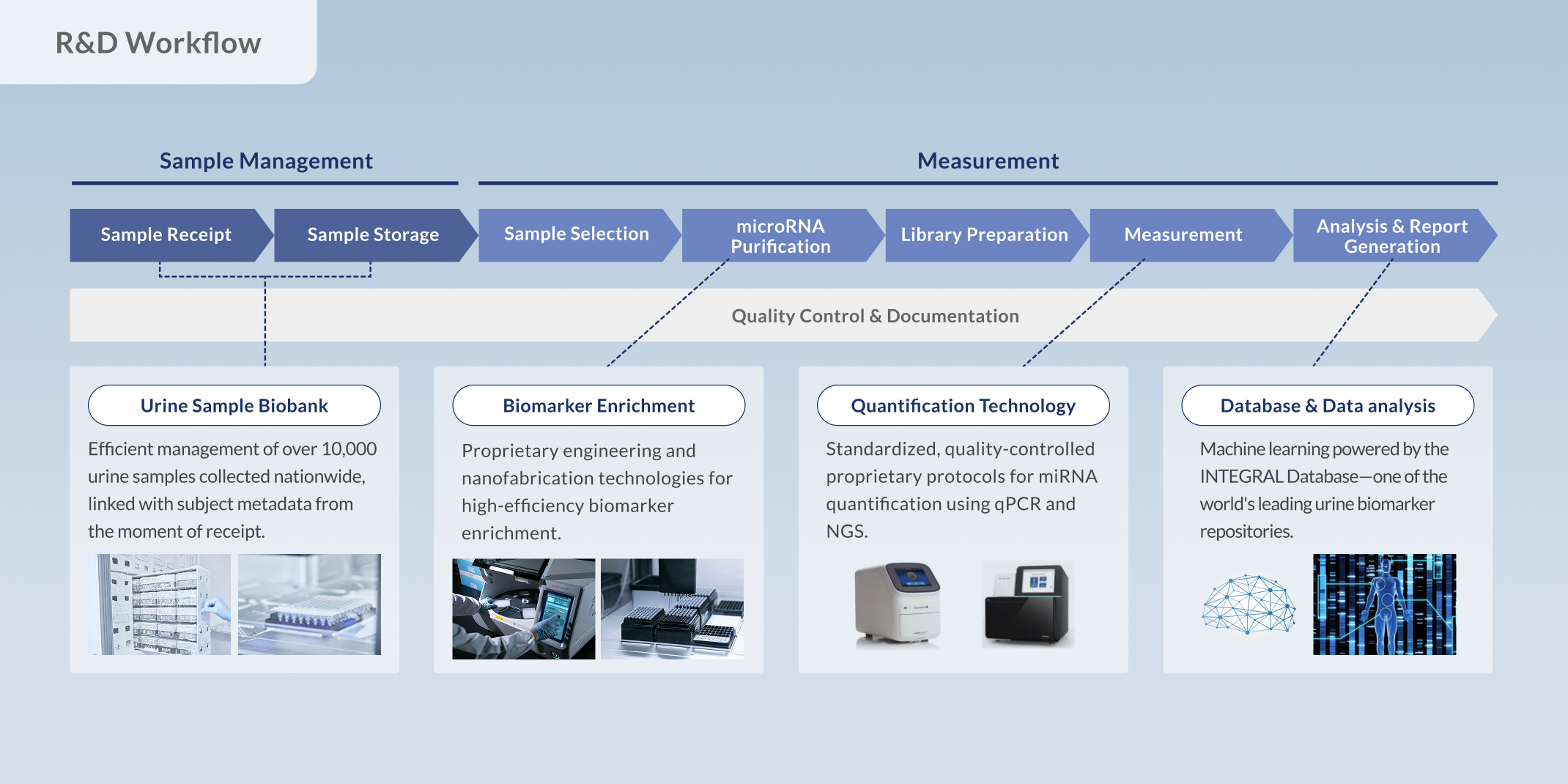
It involves generating high-quality biological data from bodily fluids such as blood or urine, or from cells and tissues, and using these data to build high-precision AI systems that directly drive innovation in diagnostics, testing, and drug discovery.
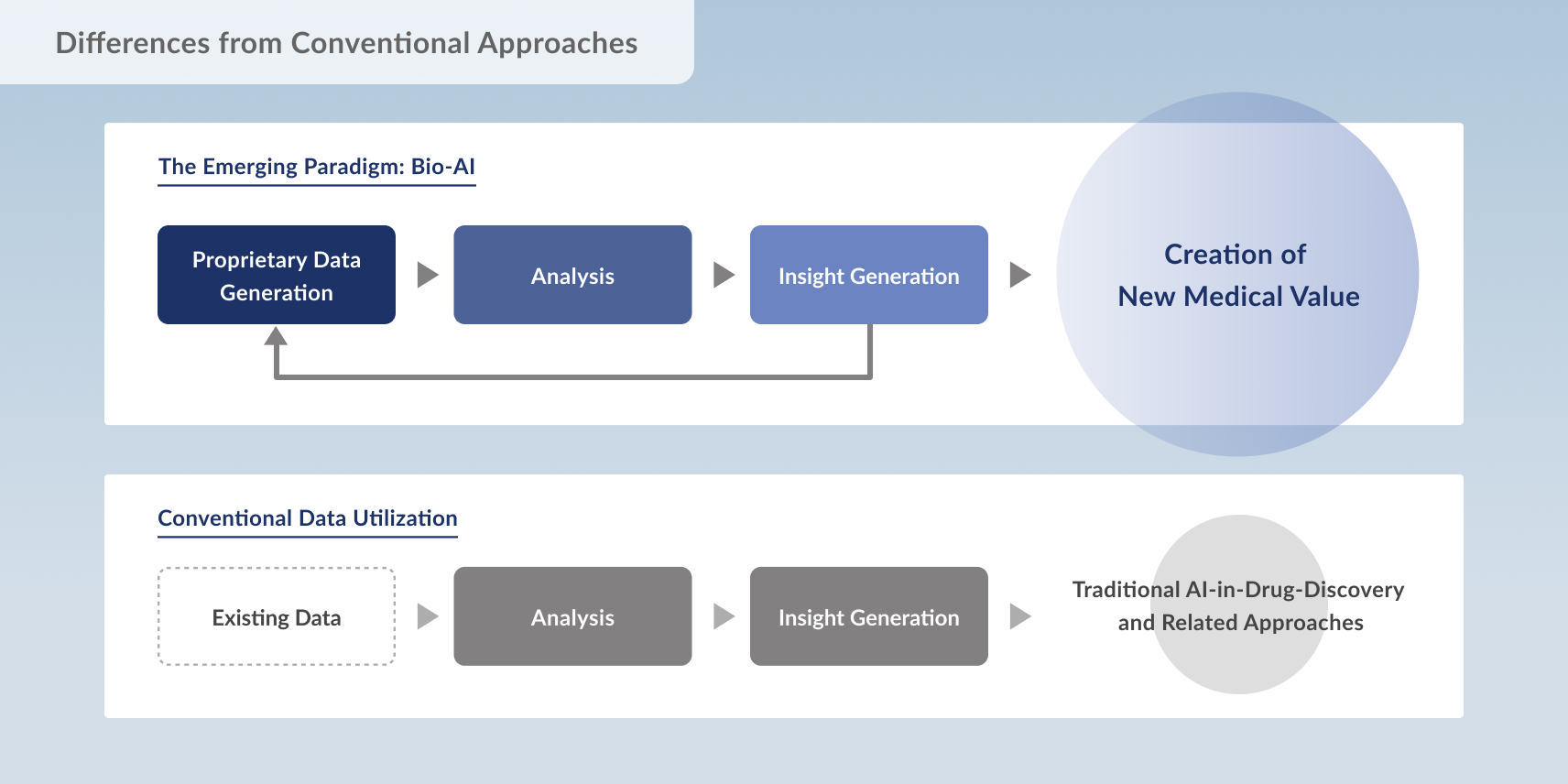
Traditionally, data utilization and AI development in biology have relied heavily on existing public datasets.
However, biological data in the real world are inherently noisy — affected by sample collection conditions, individual variability, and measurement techniques.
To interpret such data correctly and use them meaningfully, a deep understanding of biology is essential.
The emerging field of Bio-AI takes a fundamentally different approach:
it designs measurement systems, quality control, and data generation processes with the end goal of data utilization in mind, refining the biological data itself into a form optimized for machine learning.
Only after that foundation is built are data analysis, interpretation, and algorithm development carried out — enabling AI to perform at its true potential.
This approach operates through a continuous, high-speed loop:
1. Generate proprietary, high-quality biological data
2. Analyze and interpret using rigorously validated datasets
3. Feed insights back into data generation and experiment design
By accelerating this cycle, Bio-AI is redefining what’s possible in medical innovation — creating a new norm in diagnostics and drug discovery.
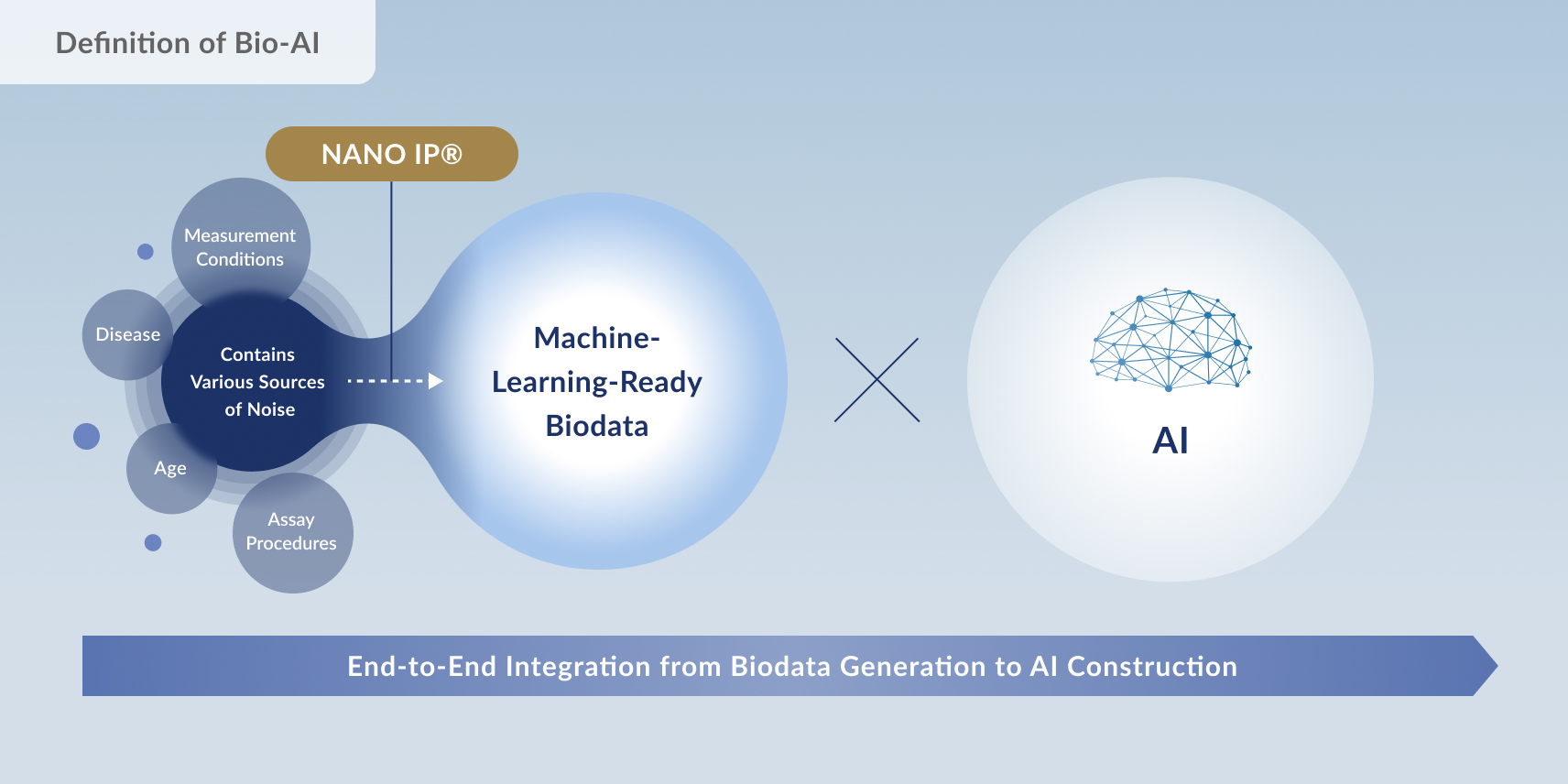
Craif integrates its proprietary NANO IP®︎ biomarker analysis platform — which extracts minute biomarker signals such as urinary microRNAs with precision and machine-learning-grade quality — with its in-house AI development framework.
By seamlessly managing every stage from data generation to AI construction, Craif accelerates the practical realization of medical value, including the ultra-early detection of cancer.
Craif’s proprietary NANO IP®︎ is a world-leading technology that enables high-precision analysis of biological signals—such as DNA and RNA—found in body fluids like urine.
This platform integrates advanced bioinformatics and large-scale data management capabilities, allowing for the comprehensive and efficient analysis of a wide range of biomarkers, including microRNAs.
By leveraging this cutting-edge technology, Craif is accelerating research aimed at detecting cancer at asymptomatic stages and identifying previously unrecognized diseases with greater speed and accuracy.
Looking ahead, NANO IP®︎ is expected to become a foundational platform for truly personalized medicine, tailored to each individual’s unique biological profile.
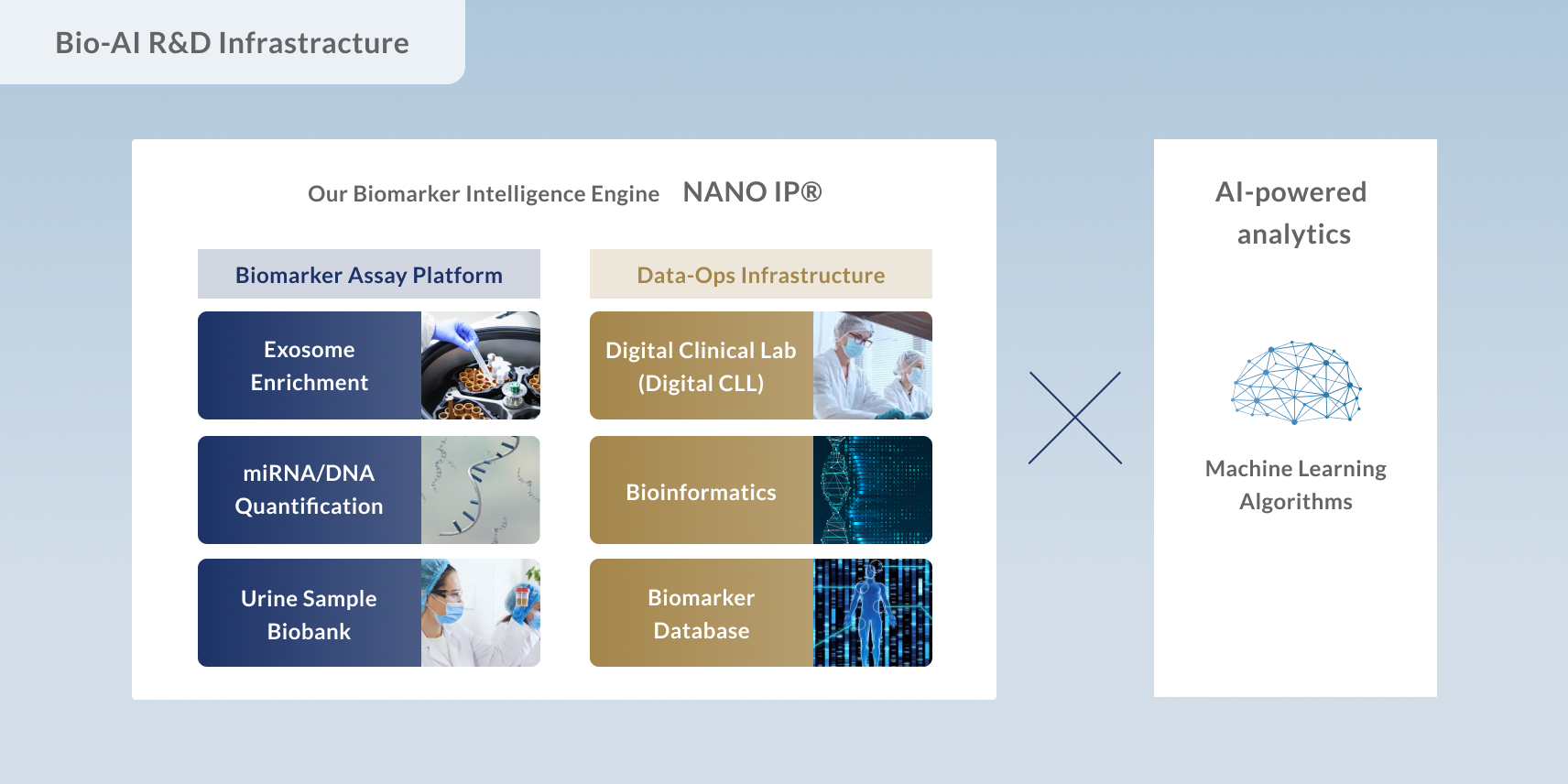
Craif’s technological foundation began with the development of a microfluidic device capable of precisely capturing exosomes—microscopic vesicles found in urine that carry valuable biological information.
By combining nanoscale engineering with precision fluid control, Craif has established a unique capability to extract disease-related biomarkers—tiny molecular “signals” that indicate the presence or progression of illness—with high accuracy.
This technology is designed to reliably detect subtle biological signs that have previously gone unnoticed, enabling a new era of early and precise diagnostics.
At Craif, we utilize cutting-edge next-generation sequencing (NGS) technology to thoroughly analyze DNA and RNA present in body fluids. This enables us to generate high-quality testing data by minimizing measurement bias and ensuring comprehensive coverage.
The data we produce serves as training data for our AI algorithms, allowing them to assess cancer risk with higher accuracy and reliability.
Accurately capturing large-scale biological data and using it to power precise cancer detection algorithms—this is the core strength of Craif’s technology.
Craif’s data analysis team includes specialists in bioinformatics and statistical analysis, supported by a structure that integrates both wet-lab and dry-lab capabilities—a key organizational strength.
By analyzing a dataset of more than 30,000 clinical samples, the team is working to elucidate the functions and regulatory mechanisms of disease-related biomarkers, enabling deeper insights into the molecular dynamics of various conditions.
Craif has built one of the world’s most extensive databases specializing in urine-derived biomarkers, integrating over 30,000 clinical samples, comprehensive nucleic acid expression profiles (including microRNAs), and detailed demographic data such as age and sex.
This robust data infrastructure enables multi-dimensional biomarker discovery and validation across a wide range of diseases, including cancer.
In collaboration with over 50 medical institutions across Japan—including university hospitals and government-designated cancer centers—Craif has collected and curated a library of more than 10,000 clinical urine samples.
This diverse sample set, reflecting a wide range of patient backgrounds, enables the development of AI models with reduced bias and enhanced generalizability.
Craif’s Digital Clinical Lab is an advanced facility that seamlessly integrates AI-driven testing and data analysis. Leveraging our proprietary Oyster® system, the entire workflow is fully digitized and automated—ensuring transparency, reproducibility, and traceability through real-time parameter monitoring.
Based at the Craif Chubu Testing Center, which was officially registered as a clinical laboratory in 2022, the lab performs high-precision analysis of the miSignal series.
With minimal human intervention, we deliver high-quality diagnostic insights quickly and accurately, enabling a new standard of clinical reliability and scalability.