Spinal Muscular Atrophy (SMA) is a severe genetic disorder that affects the nervous system and muscles. It is typically diagnosed in infancy or early childhood, causing progressive muscle weakness that impairs motor development, including the ability to roll over, walk, or even breathe and swallow. Without timely and appropriate treatment, SMA can be life-threatening.
While new treatments have emerged in recent years that offer the possibility of improving motor function, it is well known that starting treatment after symptom onset has limited effectiveness. This makes early detection—ideally before symptoms appear—critically important.
Conventional screening tests for Spinal Muscular Atrophy (SMA) typically require a blood sample to be sent to a specialized external lab for analysis using advanced equipment. As a result, it often takes 1 to 2 weeks to receive results—delaying critical treatment decisions during a vital window of opportunity.
To address this, Craif has developed a novel, rapid, and non-invasive SMA screening kit that can be used immediately after birth, providing results within just 1.5 hours—right at the point of care.
We have already launched a clinical study in Japan using the kit in real-world newborn settings to evaluate its accuracy and usability. Plans are underway to further expand the study to a larger population.

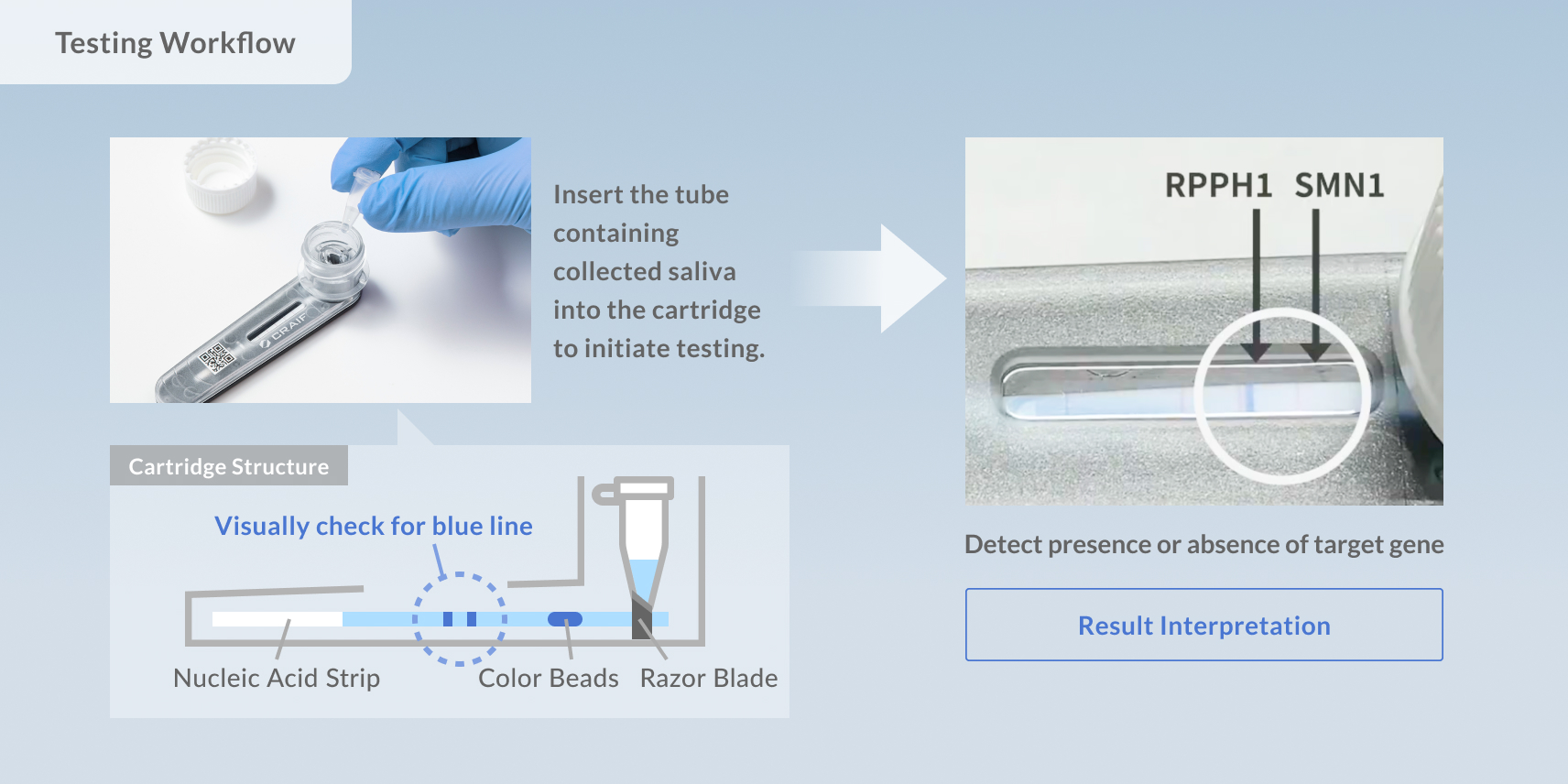
This screening kit incorporates Craif’s proprietary technology that enables the rapid detection of specific genetic sequences (nucleic acids).
Originally developed for SMA, this innovative technology is designed as a scalable platform with the potential to be applied across a broad spectrum of genetic and congenital disorders—paving the way for earlier and more accessible screening solutions.