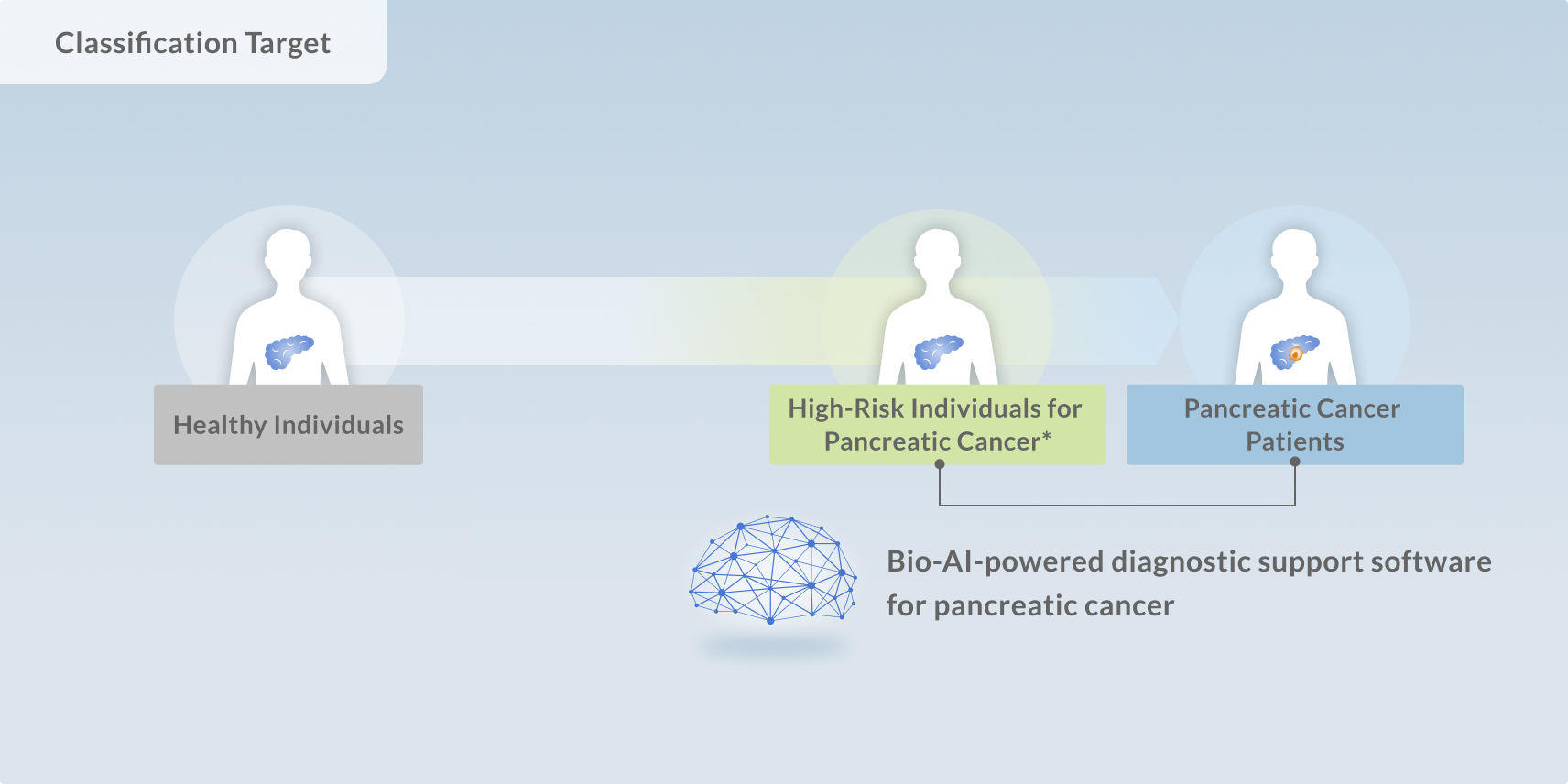
Craif is currently conducting a multicenter clinical study to validate a new medical technology that analyzes microRNAs in urine using AI to support the diagnosis of pancreatic cancer.
This trial represents a critical step toward obtaining regulatory approval from Japan’s Ministry of Health, Labour and Welfare (MHLW) and bringing this technology into clinical use as a regulated medical device.
The study involves participants, including patients diagnosed with pancreatic cancer, individuals at high risk, and healthy controls. By analyzing urine-derived microRNA profiles along with standard clinical data, the study aims to evaluate how accurately Craif’s AI can detect pancreatic cancer—particularly at an early stage.
Through this research, Craif is working to establish a novel diagnostic tool that can identify pancreatic cancer earlier and more precisely than ever before.
In collaboration with Hokkaido University Hospital and Iwanai Kyokai Hospital, Craif conducted a lung cancer screening study using its miSignal Scan technology, targeting 100 residents of Iwanai and Yoichi towns in Hokkaido—areas known for low cancer screening participation.
This was a prospective observational study designed to assess the capability of urine-based microRNA profiling for early lung cancer risk detection, while also tracking participants’ incidence rates and clinical outcomes over time.
In this clinical study, the miSignal Scan identified a lung cancer risk in a participating resident, leading to the successful removal of a stage 0 (ultra-early) lung cancer.
This study marks an important step toward validating the role of next-generation cancer screening in community health. Craif will continue expanding its efforts to assess how this technology can be implemented as a novel and accessible option for cancer screening nationwide.
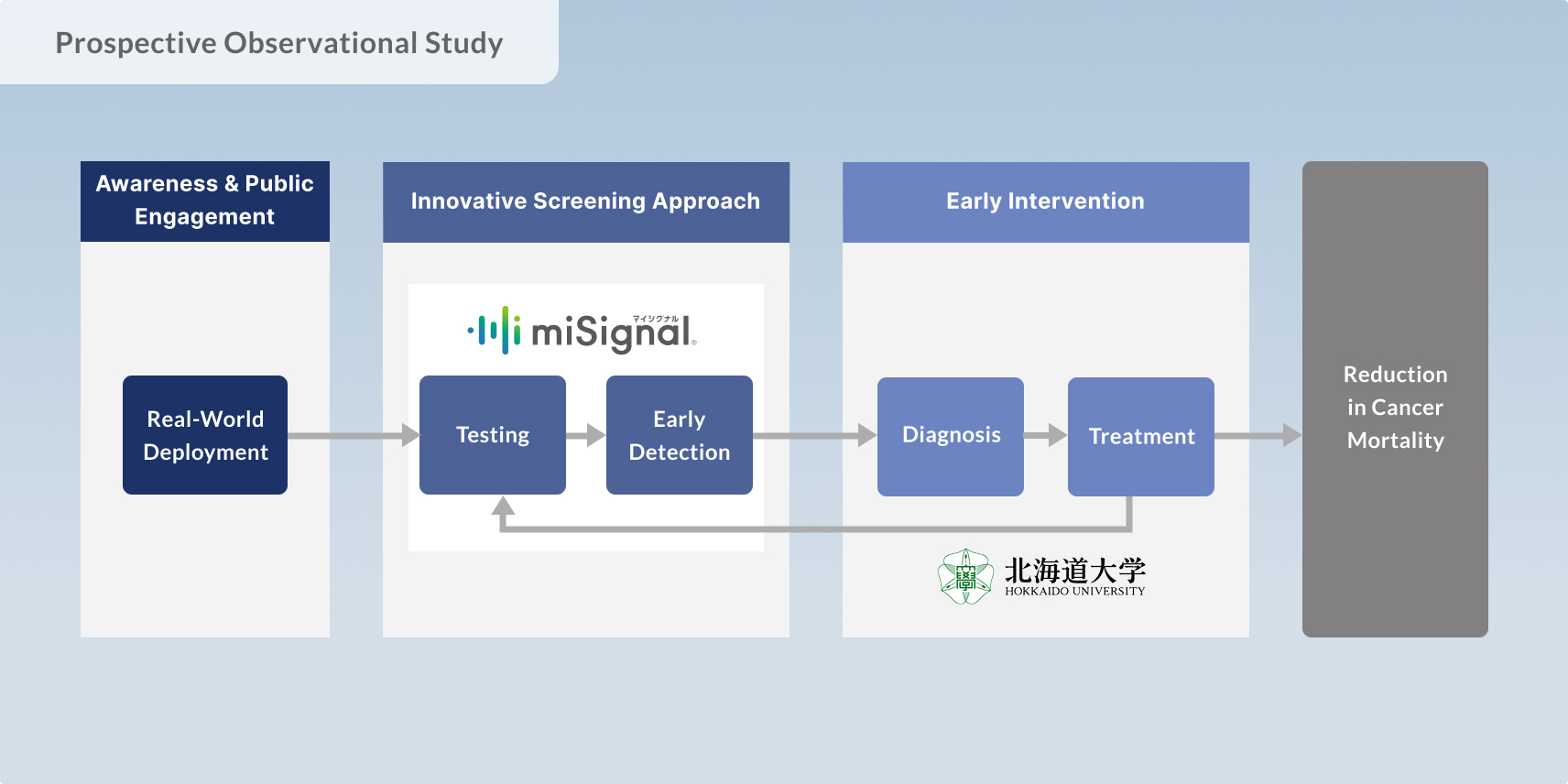
Craif, in collaboration with Nagoya University and Fujita Health University, is conducting a pilot implementation study involving 100 residents in the Tokai region of Japan. The study evaluates the feasibility of miSignal Scan, a novel urine-based screening test designed to assess pancreatic cancer risk.
This study utilizes Craif’s proprietary technology that analyzes microRNAs in urine samples using AI-based algorithms, aiming to determine how early and accurately pancreatic cancer signals can be detected. In addition to assessing diagnostic performance, participants are being followed longitudinally to investigate how test results correlate with cancer incidence and overall health outcomes.
Pancreatic cancer is notoriously difficult to detect early due to its lack of specific symptoms in the initial stages, and no effective mass screening method has been established to date. This initiative represents a critical first step toward the social implementation of a world-first, non-invasive screening approach that uses only urine to identify early cancer signals—and is being conducted in conjunction with public awareness campaigns to address this pressing unmet need.