Improving cancer survival rates by making early detection the norm.
Focusing on microRNAs—key regulators in the onset and progression of cancer—we have developed and now offer miSignal Scan*, a urine-based cancer screening test powered by our proprietary Bio-AI technology. This test enables the detection of cancer-specific signals through AI, even before symptoms appear, making truly early detection possible.
We are also developing a medical device leveraging Bio-AI for the early detection and accurate diagnosis of pancreatic cancer, one of the deadliest cancers with no effective early screening method to date. This program is being advanced in both Japan and the United States, providing a new option for identifying this notoriously hard-to-detect disease at an early stage.
* Available only in Japan


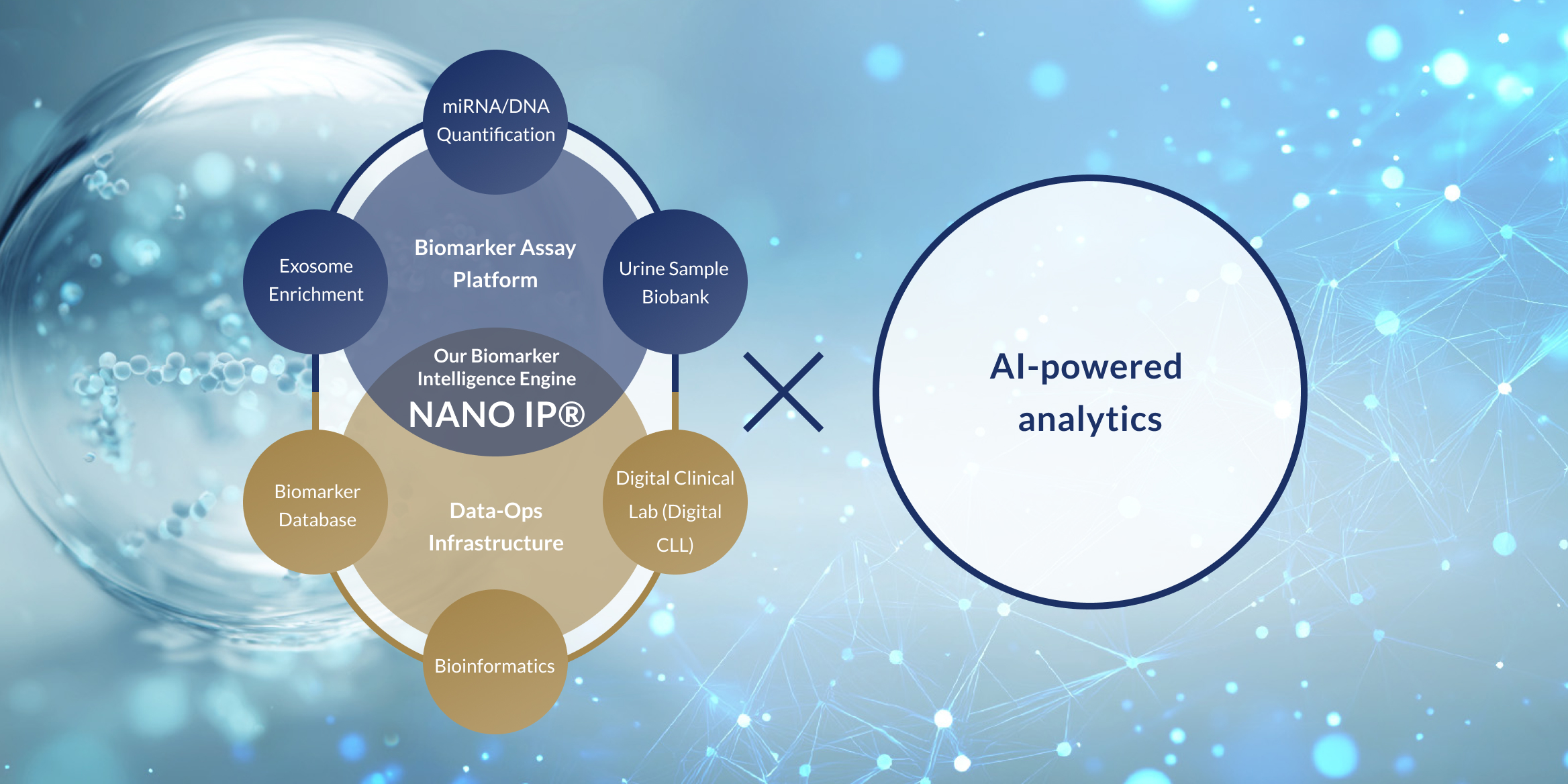
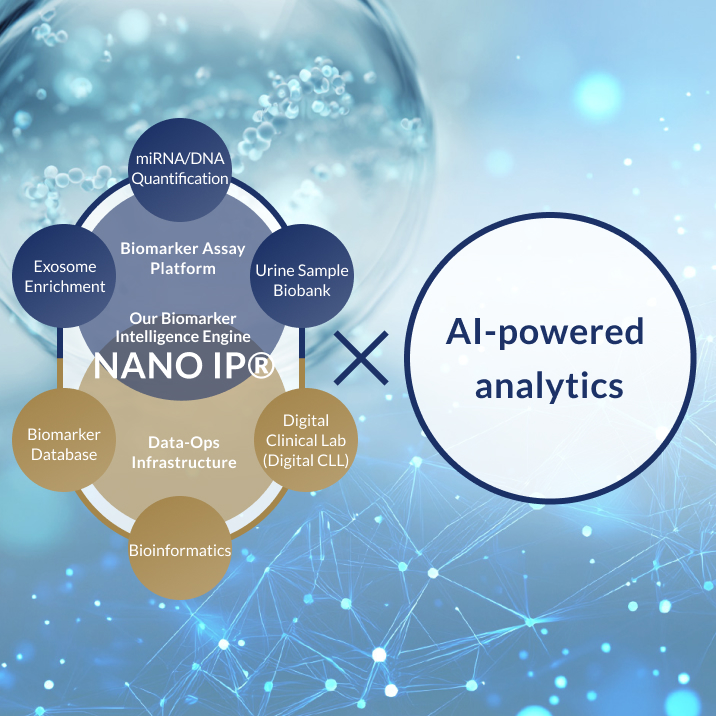
Through our proprietary biomarker analysis platform NANO IP® (NANO Intelligence Platform®)—which decodes a wide range of biological signals such as DNA and RNA found in body fluids like urine—and by combining it with advanced AI algorithms, Craif is pioneering a Bio-AI approach.
This integrated technology enables us to develop next-generation diagnostic tests and medical devices aimed at the early detection of diseases such as cancer, as well as the realization of personalized treatment tailored to each individual.